Abstract
Introduction: Patient-reported outcomes (PROs) help inform treatment decisions and enhance quality of care in patients with cancer. A phase 2 study of pembrolizumab for the treatment of relapsed/refractory classical Hodgkin Lymphoma (r/r cHL) demonstrated that the majority of patients experienced maintenance and/or improvement in disease-related symptoms, functioning and health status, especially among those who responded to treatment. We present health-related quality of life (HRQoL) outcomes in the following subgroups of r/r cHL patients: 1.) those who received autologous stem cell transplantation (ASCT) and subsequent brentuximab vedotin (BV) therapy and 2.) those who were ineligible for ASCT due to chemoresistance (no response to salvage chemotherapy) and had failed BV therapy.
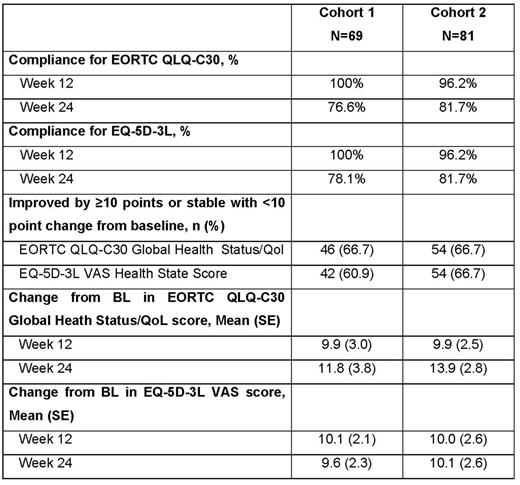
Methods: Analyses were based on data from a multicenter, single-arm, phase 2 study (ClinicalTrials.gov, NCT02453594) of pembrolizumab 200 mg administered every 3 weeks to patients with r/r cHL. HRQoL was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire - Core 30 (EORTC QLQ-C30) and the EuroQoL Five Dimensions Questionnaire 3-level version (EQ-5D-3L) instruments. Questionnaires were administered for the first five (3-week) cycles and then every 12 weeks thereafter until disease progression, death, or withdrawal. The outcomes examined were rates of PRO compliance, mean score change from baseline (BL) to weeks 12 and 24, and incidence of and time to first onset of a deterioration of ≥10 points in score from BL to weeks 12 and 24. All enrolled subjects who had received at least one dose of study medication and had completed at least one assessment were included in the analysis. There was no imputation for missing data.
Results: 150 patients were included (Table). Across the 2 cohorts, the proportion of patients that had the following patient characteristics were: age ≥65 years (0% and 18.5% respectively), male gender (52.2%, 53.1%), and ECOG performance status 0 (42.0%, 54.3%). High rates of compliance were observed across both cohorts at weeks 12 (≥96%) and 24 (≥76%) (Table). The mean increases in EORTC QLC-C30 global health status/QoL scores and EQ-5D-3L VAS scores from BL to weeks 12 and 24 were approximately 10 points or more (Table), changes that may be considered clinically meaningful. Across both cohorts, the EORTC QLQ-C30 mean symptom scale score improved by 10 or more points for fatigue and dyspnea at Week 12, and for fatigue, pain, dyspnea and insomnia at Week 24. Consistently in both cohorts, over 60% of patients had either improved (≥10 points of improvement from BL) or stable (<10 points change from BL) in their HRQoL when compared with baseline. The effects were generally consistent across patient subgroups defined by age (<65 vs. >65 years), gender, ECOG performance (0 vs. 1-2), region (Europe vs. Non-Europe), prior lines of therapy (<3 vs. >3), time to relapse since ASCT failure (<12 months vs. >12 months), race (White vs. Non-White), and refractory/relapse status (refractory vs. relapsed after >3 lines of therapy).
Conclusion: Net improvements from BL to weeks 12 and 24 in EORTC QLQ-C30 and EQ-5D-3L quality of life scores were observed in patients with r/r cHL during treatment with pembrolizumab.
Support: Merck & Co., Inc., Kenilworth, New Jersey, USA
von Tresckow: MSD: Consultancy, Research Funding; Takeda: Consultancy, Other: travel, housing, congress fees, Research Funding; Novartis: Honoraria, Other: travel, housing, congress fees, Research Funding; BMS: Other: travel, housing, congress fees. Fanale: GENENTECH: Research Funding; TAKEDA: Honoraria, Research Funding; ONYX: Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CELGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; SEATTLE GENETICS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MERCK: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC THERAPEUTICS: Research Funding; MOLECULAR TEMPLATES: Research Funding. Ardeshna: Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: conference expenses; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: conference expenses; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: conference expenses, Research Funding, Speakers Bureau. Chen: Genentech: Speakers Bureau; Seattle Genetics: Consultancy, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Pfizer: Consultancy; Merck: Consultancy, Speakers Bureau; Affimed: Research Funding. Meissner: BMS: Other: Non-Financial Support; Takeda: Other: Non-Financial Support; Celgene: Other: Non-Financial Support; Amgen: Other: Non-Financial Support. Morschhauser: Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Gilead: Consultancy; Servier: Consultancy; Bristol-Myers Squibb: Consultancy, Honoraria. Moskowitz: Merck: Consultancy, Research Funding; Genentech BioOncology: Consultancy; Pharmacyclics: Research Funding; Seattle Genetics: Consultancy, Other: Ad Board, Research Funding; Celgene: Consultancy. Zinzani: Celgene, Roche, Janssen, Gilead, Takeda, BMS, MSD, Servier, Sandoz, Mundipharma: Honoraria; Celgene, Janssen, Gilead, Roche, Takeda, BMS, MSD, Sandoz, Servier, Mundipharma: Speakers Bureau; Merck: Consultancy, Other: Advisory board. Giezek: Merck & Co., Inc.: Employment, Other: stock/stock options. Balakumaran: Merck & Co., Inc.: Employment, Other: stock/stock options. Raut: Merck & Co., Inc.: Employment, Other: stock/stock options. Vo: Merck & Co., Inc.: Employment, Other: stock/stock options.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal